Description
SPECIFICATIONS
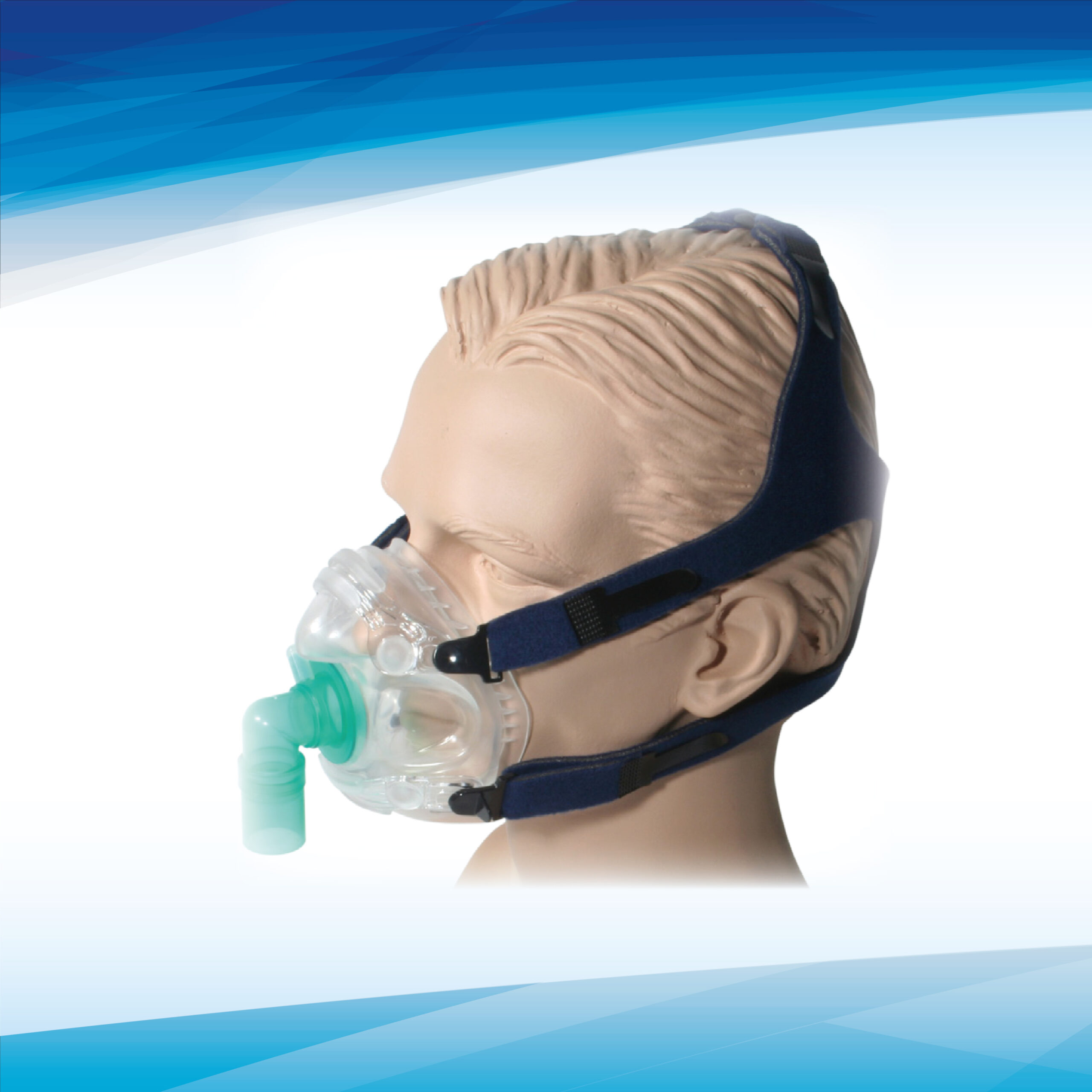
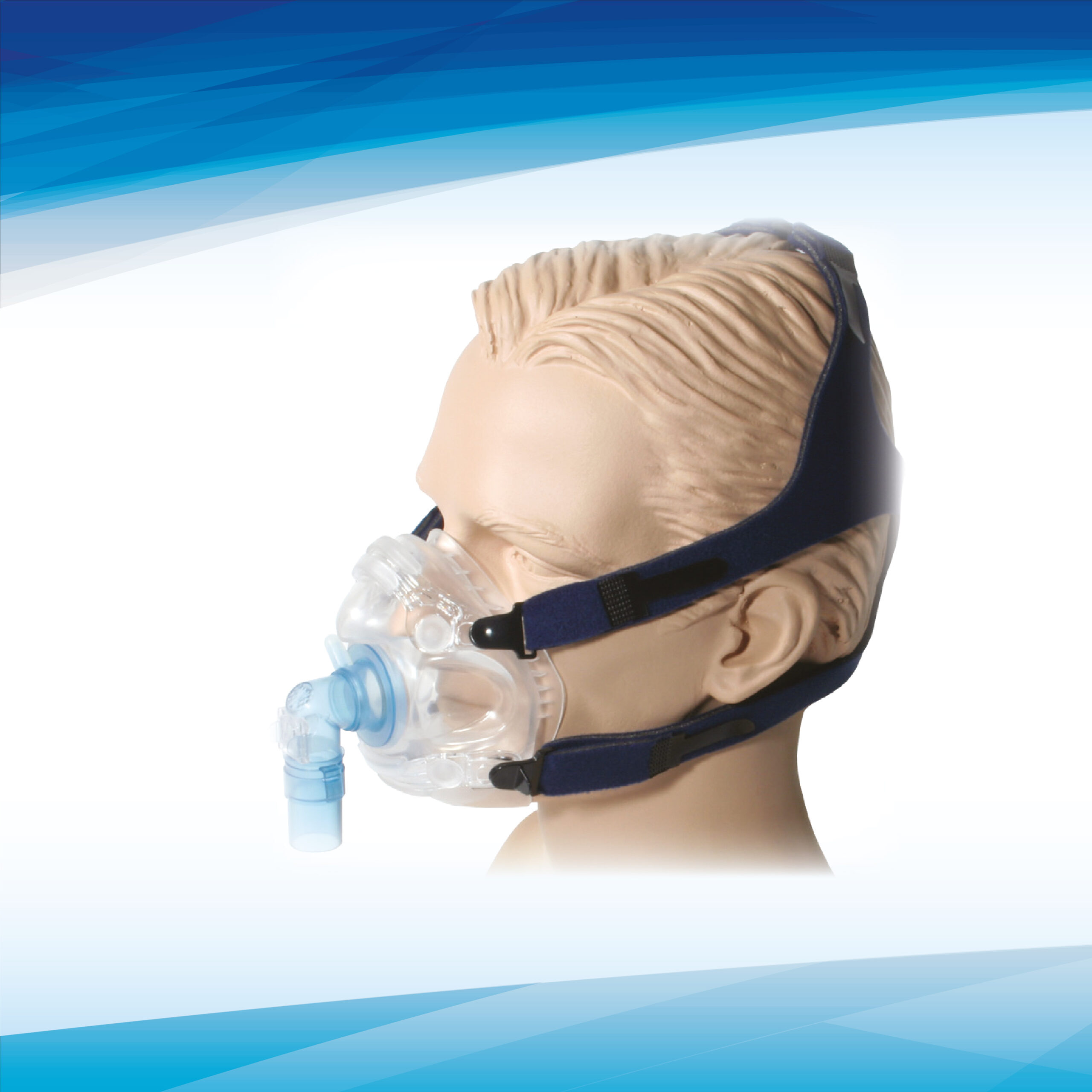
MATERIALS (See Fig. A)
Face Piece with Structural Braces Thermoplastic Elastomer, Polycarbonate Thermoplastic
Swivel Port – Mask Adapter, Elbow, 22mm Port Polycarbonate Thermoplastic, Translucent Green
Headgear Polyurethane Foam Black, Nylon UBL Black and Blue
Headgear Hook Nylon, Black
Headgear Strap Clips (4), Tri-glide Polypropylene, Black
GENERAL INFORMATION
• Intended Use: The 6500 Series V2 Masks™ are disposable, single-patient-use, adult oro-nasal masks intended to provide a patient interface for application of noninvasive ven-tilation. The masks are to be used as an accessory to continuous ventilators which have
adequate alarms and safety systems for ventilator failure.
• Indications for Use & Environment: The masks are specifically indicated for use on adult patients (greater than 30 kilograms weight) to administer positive pressure ventilation for treatment of respiratory failure or respiratory insufficiency. They are intended for use
on patients who are appropriate candidates for noninvasive ventilation, in the hospital, or other clinical settings by individuals that have received at least minimal instruction or
training on the use of the masks as well as the device and system to which the masks are intended to connect.
• Cautions:
a.) Federal law restricts this device to sale by or on the order of a physician.
b.) Patients with facial hair, especially beards, may experience mask leakage whichmay require shaving the hair.
• Warnings: Refer to: Instructions for Use, Document No. 691274, shipped with product.
• Contraindications & Complications: Refer to: Instructions for Use, Document No.
691274, shipped with product.